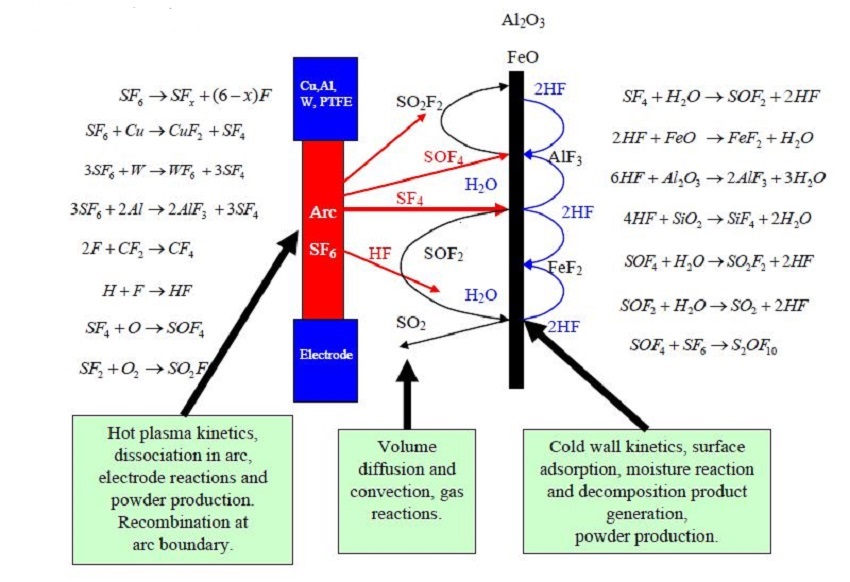
During normal load or short-circuit current switching, SF6 molecules are ionized and fragmented by the arc.
Figure show the main reaction processes and where each is likely to occur. SF4, as the main decomposition product from the electric discharge, first reacts with H2O on the inner wall surface resulting in SOF2. The metal fluorides remain as powder or dust on the surface. H2O is released in this reaction and therefore available for further reactions with SF4 or for the much slower conversion of SOF2 to SO2 . Since in this process, H2O is in fact not consumed but plays the role of a catalyst. The factors involved are:
- Formation rate of decomposition products as a function of the arc or discharge energy.
- Transport speed (diffusion or convection) of decomposition products from the arc or discharge location to the walls of the compartment.
- Gas mixing of decomposition products created at surfaces (diffusion or convection)
- Reaction rate of decomposition products at wall surfaces
- Adsorption rate of decomposition products in adsorber materials depending on the properties and the location of the absorber material



