Global warming will be a major issue in the future then using SF6 for switching equipment and high consumption of SF6 gas will decrease in the future
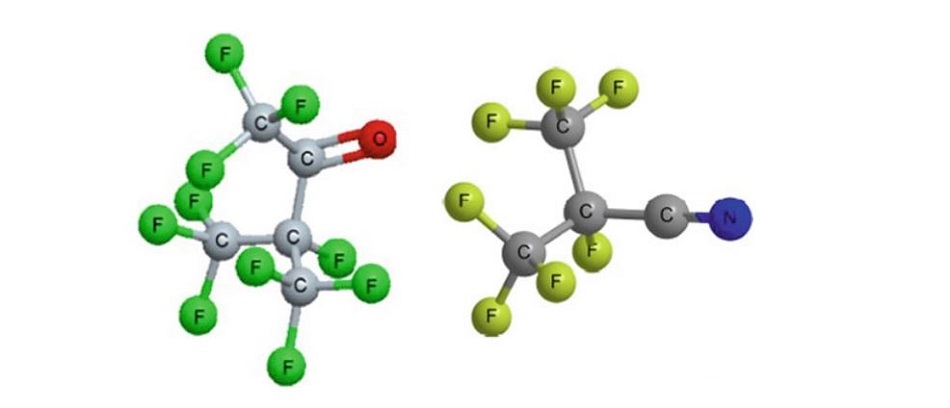
Fig show fluoroketone (left) and fluoronitrile (right) molecular structures. They both have a carbon backbone, with 5 and 4 atoms, respectively.
Both the fluoroketone and fluoronitrile have a substantially higher dielectric strength than SF6, about a factor 2 at the same pressure. The important factor is that these gases must be mixed with a buffer to be able to use them at low temp; otherwise, they condense to the liquid state with a very low vapour pressure and thus show a poor dielectric strength and poor current interrupting performance. Several carrier gases have been proposed such as air,nitrogen,oxygen,CO2
Finding an appropriate concentration of fluoroketone or fluoronitrile in the carrier gas, as well as the total gas pressure becomes a trade off between the dielectric strength obtained and usable temp range.
A high partial pressure of fluoroketone or fluoronitrile and a high total gas pressure give a sufficient dielectric strength, but cannot cover the entire temperature range usually specified for outdoor CB



